
Adnane Achour
Karolinska Institutet, Sweden
Title: Rigidification of MHC-I/peptide complexes is an essential step for enhanced T cell recognition of cancer-associated neo-epitopes associated with impaired processing
Biography
Biography: Adnane Achour
Abstract
MHC-I down-regulation represents a significant challenge for T cell-based immunotherapy. T cell epitopes associated with impaired peptide processing (TEIPP) constitute a novel category of immunogenic neo-antigens that are selectively presented on TAP-deficient cells. TEIPP neo-epitopes are CD8 T cell targets, derived from non-mutated self-proteins. We recently evaluated thymus selection and peripheral behavior of TEIPP-specific T cells and demonstrated that TEIPP-specific T cells in TAP-deficient mice are deleted by central tolerance, while the same T cells in WT mice are naive and sustain. Thus the results of this study suggest that TEIPPs have potential to be successful targets for eliminating MHC-low tumors and reduce cancer immune escape. The crystal structure of H-2Db in complex with the first identified TEIPP antigen Trh4 (MCLRMTAVM) demonstrated that, in contrast to prototypic H-2Db peptides, Trh4 takes a non-canonical binding pattern with extensive sulfur-p interactions. Importantly, the non-canonical methionine at peptide position 5 acts as a main anchor, altering the conformation of H-2Db residues and thereby forming a unique MHC/peptide conformer that is essential for recognition by TEIPP-specific T cells. We have previously demonstrated that modification of peptide position 3 to a proline in H-2Db-binding peptides increases significantly the overall stability of MHC-I/peptide complexes and the immunogenicity of endogenous T cells towards cancer-associated epitopes. The results demonstrate that vaccination with Trh4-p3P induced significant CTL responses towards Trh4+-cancer target cells. Importantly, our results stand in strong contrast to the current dogma that stipulates that higher immunogenicity is directly linked to higher MHC/peptide complex stability. Indeed, although much more immunogenic, the H-2Db/Trh4-p3P complex is clearly less stable than H-2Db/Thr4. Instead, rigidification of Trh4 and the α2 helix in H-2Db is directly responsible for enhanced TCR recognition. Our results reveal the importance of the rigidification of the MHC/peptide complex for enhanced T cell recognition.
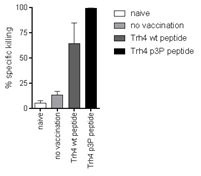
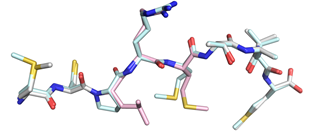
Left. Vaccination with Trh4-p3P induces significantly enhanced recognition of cancer target cells displaying the wild-type peptide. Right. Superposition of the crystal structures of H-2Db/Trh4 and H-2Db/Trh4-p3P demonstrates high conformational similarities.
References:
1. Van Stipdonk et al (2009) Design of agonistic altered peptides for the robust induction of CTL directed towards H-2Db in complex with the melanoma-associated epitope gp100. Cancer Research, 69, 7784-7792.
2. Uchtenhagen et al (2013) Proline substitution independently enhances H-2Db complex stabilization and TCR recognition of melanoma-associated peptides. European Journal of Immunology, 43(11):3051-3060.
3. Doorduijn et al (2016) Absence of central tolerance enables therapeutic exploitation of a CD8 T cell subset targeting hidden self-antigens. Journal of Clinical Investigation, 126(2): 784-794.
4. Hafstrand et al (2016) The MHC class I cancer-associated neo-epitope Trh4 linked with impaired peptide processing reveals a unique non-canonical TCR conformer. Journal of Immunology, 196(5):2327-34.
5. Kiessling (2016) TAP-ing into TEIPPs for cancer immunotherapy. Journal of Clinical Investigation, 126(2):480-2.
